Your Which element has the most metallic character images are ready. Which element has the most metallic character are a topic that is being searched for and liked by netizens today. You can Get the Which element has the most metallic character files here. Download all free images.
If you’re searching for which element has the most metallic character pictures information related to the which element has the most metallic character topic, you have pay a visit to the right site. Our website always provides you with hints for refferencing the highest quality video and picture content, please kindly search and locate more enlightening video articles and graphics that fit your interests.
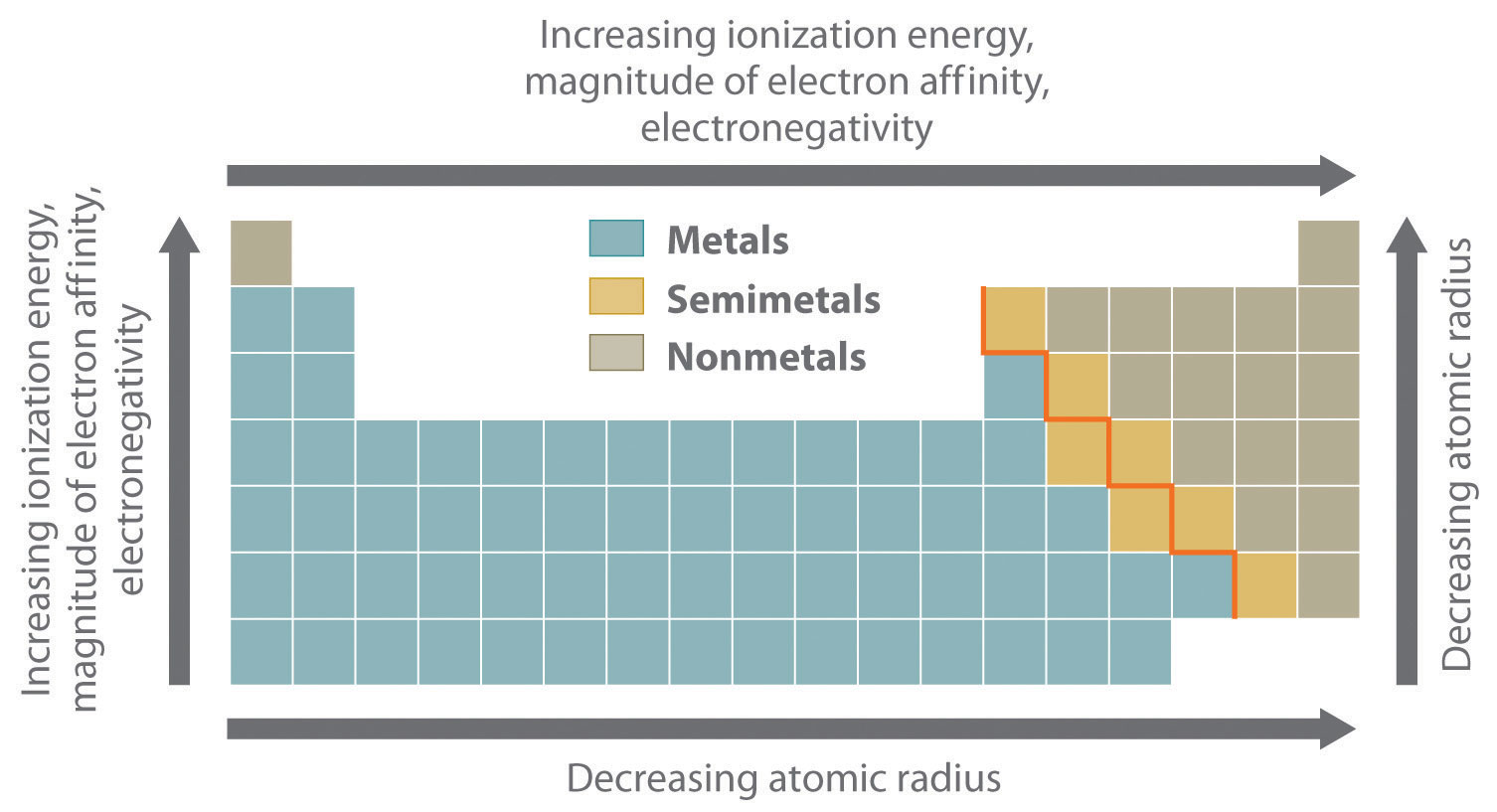
Which Element Has The Most Metallic Character. This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell. Sodium Na is the leftmost among the choices which makes it the most metallic. Bismuth Bi is a metallic element. Use the periodic table to predict which element has the most metallic character.
 3 4 Trends In Electron Affinity And Metallic Character Chemistry Libretexts From chem.libretexts.org
3 4 Trends In Electron Affinity And Metallic Character Chemistry Libretexts From chem.libretexts.org
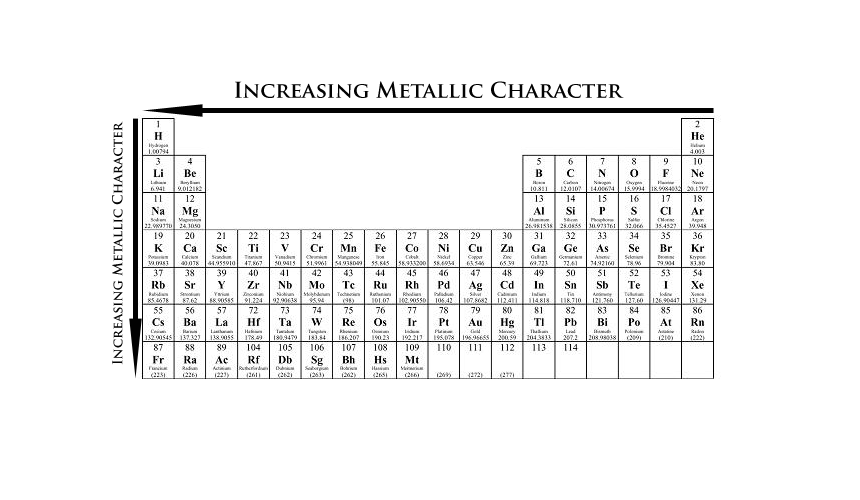
Boron is the first member of the group and Thallium is the last Answer the following questions in relation to the above group of elements. Metallic character depends on the ability of an element to lose its outer valence electrons. Examples of properties related to metallic character include thermal and electrical conductivity metallic luster hardness ductility and malleability. Bi is a poor metal it is similar to the arsenic and antimony. Use the periodic table to predict which element has the most metallic character. Francium element with highest metallic character cesium next highest level of metallic character sodium copper silver iron gold aluminum Alloys and Metallic Character Although the term metallic character is typically applied to pure elements alloys may also display metallic character.
Francium is the most metallic element of all man-made elements Cesium is the most metallic element of all natural elements.
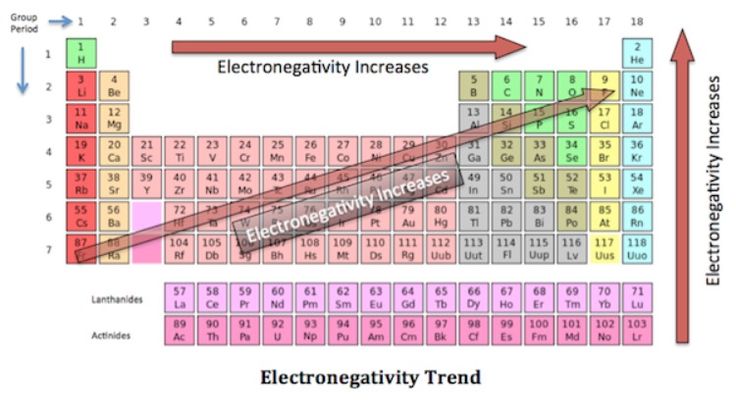
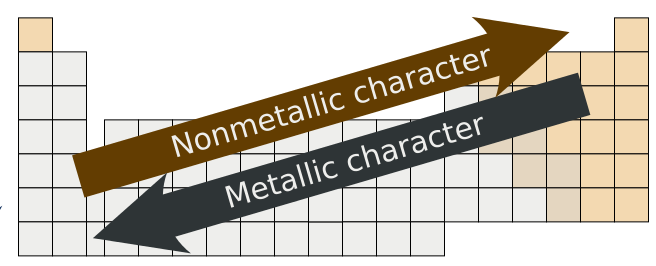
The natural element with the highest metallic character is cesium which is found directly above francium on the periodic table. Francium is the most metallic element of all man-made elements Cesium is the most metallic element of all natural elements. Metallic character increases as you move down an element group in the periodic table. Which element has highest metallic character. Metallic characteristics decrease from left to right across a period. Which element has most metallic character.
 Source: 170188733453308075.weebly.com
Source: 170188733453308075.weebly.com
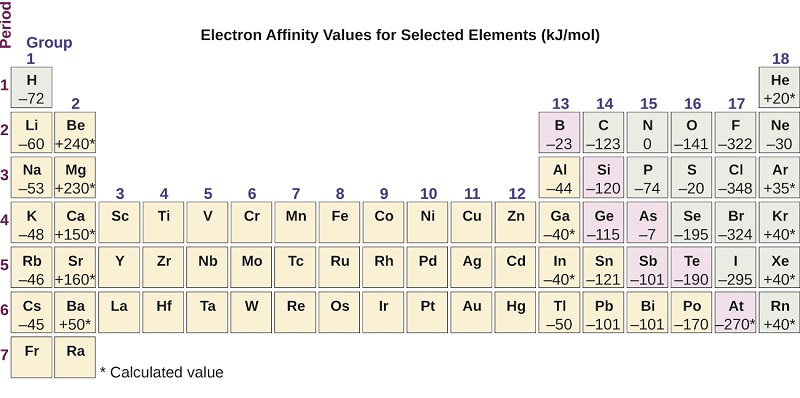
Which element has the most metallic character. FluorineWe found cesium strontium aluminum sulfur chlorine and fluorine on the periodic table. CesiumThe most metallic element is francium. Metallic character increases as you move down an element group in the periodic table. Use the periodic table to predict which element has the LEAST metallic character.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Boron Aluminium Gallium Indium Thallium are elements in the Periodic Table. Aluminium indium and thallium are the members of the boron family. The natural element with the highest metallic character is cesium which is found directly above francium on the periodic table. However francium is a man-made element except for one isotope and all isotopes are so radioactive they almost instantly decay into another element. Metallic character decreases as you move across a period in the periodic table from left to right.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Francium is extremely rare and is radioactive with the longest half-life at 22 min so there is no empirical. The elements which have a tendency to gain electrons are known as non-metals. Boron Aluminium Gallium Indium Thallium are elements in the Periodic Table. Bi is a poor metal it is similar to the arsenic and antimony. So the element at the leftmost will have the highest metallic character because metallic character decreases from left to right of the periodic table.
 Source: toppr.com
Source: toppr.com
They all follow the same periodic trends the electronegativity decreases down the group their ionization energy decreases down the group the atomic radi. Which element has most metallic character. Boron Aluminium Gallium Indium Thallium are elements in the Periodic Table. So thallium is most active metal among all. Sodium Na is the leftmost among the choices which makes it the most metallic.
 Source: byjus.com
Source: byjus.com
The natural element with the highest metallic character is cesium which is found directly above francium on the periodic table. Francium element with highest metallic character cesium next highest level of metallic character sodium copper silver iron gold aluminum Alloys and Metallic Character Although the term metallic character is typically applied to pure elements alloys may also display metallic character. Cesium is the farthest left and the lowest while fluorine is the farthest right and the highest so we know they have the highest metallic character and the lowest metallic character respectively. The elements which have a tendency to gain electrons are known as non-metals. Sodium Na is the leftmost among the choices which makes it the most metallic.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Cesium is the farthest left and the lowest while fluorine is the farthest right and the highest so we know they have the highest metallic character and the lowest metallic character respectively. The natural element with the highest metallic character is cesium which is found directly above francium on the periodic table. This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell. As we know that in a periodic table the metallic character shows a gradual increase down a group and reduces across a period. In the periodic table the metallic character decreases across a period from left to right and increases as we move down the element group.
 Source: sciencenotes.org
Source: sciencenotes.org
This is caused by the decrease in radius of the atom that allows the out. 224 views Related Answer. Bi is a poor metal it is similar to the arsenic and antimony. As the elements in Group 2 are considered in order of increasing atomic number the atomic. The elements which have a tendency to gain electrons are known as non-metals.
 Source: youtube.com
Source: youtube.com
The natural element with the highest metallic character is cesium which is found directly above francium on the periodic table. Calculate the work done force by the mass of 5kg by the changing of velocity 2m to 5ms Find the altitude of the earth satellite of its critical velocity is 5 kms. Also find its period of revolution. As an example the metallic character of Beryillium 4 would not be as great as the metallic character of Barium 56. Francium element with highest metallic character cesium next highest level of metallic character sodium copper silver iron gold aluminum Alloys and Metallic Character Although the term metallic character is typically applied to pure elements alloys may also display metallic character.
 Source: toppr.com
Source: toppr.com
Boron belongs to the 13 group of a periodic table. Which element has most metallic character. Boron belongs to the 13 group of a periodic table. The elements which have a tendency to gain electrons are known as non-metals. Which of these Group 14 elements has the most metallic properties.
 Source: online-sciences.com
Source: online-sciences.com
Sodium Na is the leftmost among the choices which makes it the most metallic. 224 views Related Answer. As an example the metallic character of Beryillium 4 would not be as great as the metallic character of Barium 56. Metallic character depends on the ability of an element to lose its outer valence electrons. Which of these Group 14 elements has the most metallic properties.
 Source: sciencenotes.org
Source: sciencenotes.org
Francium is extremely rare and is radioactive with the longest half-life at 22 min so there is no empirical. Boron is the first member of the group and Thallium is the last Answer the following questions in relation to the above group of elements. The elements which have a tendency to gain electrons are known as non-metals. Cesium is the farthest left and the lowest while fluorine is the farthest right and the highest so we know they have the highest metallic character and the lowest metallic character respectively. 224 views Related Answer.
 Source: api.simply.science
Source: api.simply.science
Francium is the most metallic element of all man-made elements Cesium is the most metallic element of all natural elements. Francium is the most metallic element of all man-made elements Cesium is the most metallic element of all natural elements. Cesium is the farthest left and the lowest while fluorine is the farthest right and the highest so we know they have the highest metallic character and the lowest metallic character respectively. FluorineWe found cesium strontium aluminum sulfur chlorine and fluorine on the periodic table. Francium element with highest metallic character cesium next highest level of metallic character sodium copper silver iron gold aluminum Alloys and Metallic Character Although the term metallic character is typically applied to pure elements alloys may also display metallic character.
 Source: saylordotorg.github.io
Source: saylordotorg.github.io
Class 9th - what is atoms. Which element has the most metallic character. Francium is extremely rare and is radioactive with the longest half-life at 22 min so there is no empirical. It has the lowest electronegativity and ionization. Metallic characteristics decrease from left to right across a period.
 Source: chem.libretexts.org
Source: chem.libretexts.org
Which of these Group 14 elements has the most metallic properties. Francium is the most metallic element of all man-made elements Cesium is the most metallic element of all natural elements. Which element has most metallic character. The alkali metals in group 1 are the most active metals and cesium is the last element in the group for which we have experimental data. Also find its period of revolution.
 Source: api.simply.science
Source: api.simply.science
This occurs as atoms more readily accept electrons to fill a valence shell than lose them to remove the unfilled shell. In the periodic table the metallic character decreases across a period from left to right and increases as we move down the element group. Francium is extremely rare and is radioactive with the longest half-life at 22 min so there is no empirical. It is the last element in the group which makes it the most metallic. Which element has highest metallic character.
 Source: courses.lumenlearning.com
Source: courses.lumenlearning.com
Boron Aluminium Gallium Indium Thallium are elements in the Periodic Table. Calculate the work done force by the mass of 5kg by the changing of velocity 2m to 5ms Find the altitude of the earth satellite of its critical velocity is 5 kms. Cesium is the farthest left and the lowest while fluorine is the farthest right and the highest so we know they have the highest metallic character and the lowest metallic character respectively. Metallic character increases as you move down an element group in the periodic table. Answer 1 of 19.
 Source: xaktly.com
Source: xaktly.com
Among the above options Sodium is the element with the most metallic character in the periodic table. In the periodic table the metallic character decreases across a period from left to right and increases as we move down the element group. The natural element with the highest metallic character is cesium which is found directly above francium on the periodic table. This is caused by the decrease in radius of the atom that allows the out. FluorineWe found cesium strontium aluminum sulfur chlorine and fluorine on the periodic table.
 Source: britannica.com
Source: britannica.com
As the elements in Group 2 are considered in order of increasing atomic number the atomic. As the elements in Group 2 are considered in order of increasing atomic number the atomic. Which element has most metallic character. Boron Aluminium Gallium Indium Thallium are elements in the Periodic Table. The most metallic element is francium followed by cesium.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site good, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also bookmark this blog page with the title which element has the most metallic character by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






